Glass, What a Mysterious
Substance It is.
- How glass was called in Japan?
- Giyaman from diamao in Spanish
Bowri Chinese origin
Ruri from vaidurya in Sanskrit
Biidoro from vidro in Portuguese
Garashi Used in Tottori and Shimabara provinces
Ga-su Used in Tottori province
Garansu Used in Iyo province
If you know of any other expressions, please let us know.
- Foreword:
It is said that human beings first met with glass 5,000 B.C. So that human
beings have been living with as long as 7,000 years. We think that handling
glass is one of our life time works, and we are very happy to deal with
the genuine article.
Definition
of Glass (In a narrow definition):
An Inorganic Substance available by cooling molten substances but not
crystallizing them:
(1) It is not a crystal,
(2) Made from molten substances by cooling it, and
(3) An inorganic substance.
Under this definition, the fiber glass for optical communications is considered
not to be a glass, as it is made by reactive evaporation from a vapor phase
and is not a molten substance as shown in (2) above.
(In a Wide Definition): Non-crystal Solid State
shows Glass Transformation phenomenon
1) Non-crystalline Solid
2) A Glass Transformation Phenomenon
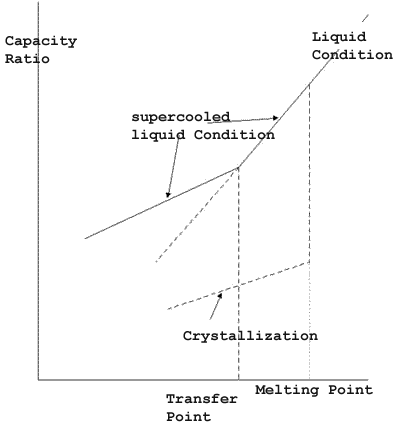
- Glass transformation means that if a glass material is heated or an
supercooled liquid is cooled, it becomes a glass, and a sudden change in
its thermal expansion coefficient and specific heat capacity at its melting
point, or two thirds or one half of its liquid phase temperature. It can
be said that glass is an supercooled liquid. (from the Glass Dictionary)
- Mystery
of Glass:
Why glass is transparent?
It is not so easy to answer that why glass (water, too) is transparent.
Generally, a substance is a composition of a group of crystals, and each
crystal has a boundary. For example, an aluminum oxide consists of particles
whose diameter is in micron meter, where the wave length of visible rays
are shorter than the diameter of the particles. So that visible rays are
scattered by hitting the aluminum oxide particles, and appear to be a color
in white. On the other hand, the particle of water is thousandth smaller
than wave length of visible rays. Therefore, visible rays are not prevented
from passing through water. Glass is made of oxidized silicon particles
of twice as big as that of water where each particle is infinitely connected
with no boundary at all. For details, refers to "garasu are kore"
authored by Toyo Keizai Shinpou Co., Ltd.
(1) The particle of glass is much smaller than that of wave length of visible
rays.
(2) No boundary due to infinite connection of oxidized silicon.
- (l)Is Glass is Solid or Liquid?
- It is a supercooled liquid. It does not exist in the natural world.
Few exceptions exist such as obsidian and meteorite glass.
- (2) Why Glass is so Fragile?
Why it is so fragile?
Is there any method available to make a non-breakable glass?
Researchers have spent many years and found that the cause of such breakage
is due to microscopic cracks on the surface of glass.
Break of glass means that the combination of silicon and oxygen is disintegrated.
But the power of such combination force is very strong.
In order to disintegrate such combination, a force of one(1) ton per square
millimeter (likely a 10,000 ton vessel on a child's head) is necessary.
However, in the real world, glass easily breaks one hundredth of such force.
This means that there are many microscopic invisible cracks on the surface
of glass, and if a cracking force is concentrated to one of those cracks,
it grows to a break force against the glass. Glass, a supercooled liquid,
cannot prevent cracks from growing since it does not have any boundary
while other solid structures have boundaries in crystal.
This is phenomenon is called:
Blue discoloring An interference fringe color.
White discoloring Deposits of Alkaline and Silicon oxide Tarnish of glass
means
this phenomenon
Kinds of Flat
Glasses Used for Generic Construction Works
- (1) Ordinary Flat Glasses
a) Transparent Glasses Colburn and Fourcourt.
b) Molded Flat Glasses Rolled out.
c) Wire Meshed Glasses Roll-out Calendar.
(2) Sheet Glasses Produced by the Floating Process
a) Transparent Glass by the Floating Process
b) Colored Glass by the Floating Process
Colburn and Fourcourt Processes
Flat glass productions by these processes are no longer
used in Japan in these days.
Although these two(2) processes enabled to produce flat
surface sheet glasses in certain extent, buffing processes were necessary
for mirrors. Still some of foreign manufacturers are using these processes.
Other Major
Manufacturing Processes of Special Type Glasses
- Engineers in the field of electronics
components are really interested in these glasses for more use in the future.
- A
Glass Production Process does not employ heat melting:
- This process enables to produce glass at a lower temperature
than conventional solution process.
Since solution is not required to be heated at a very high temperature
where crystallization occurs, a new composite glass can be produced which
could never be produced by the conventional processes.
Purification of the material is so easy, therefore, it enables to produce
very pure glass. Researchers have been challenging to produce quartz glass
and a compound glass made of inorganic and organic materials for contact
lens.
- Kinds
of Glasses Classified by their composites:
- A) Soda Calcic glass (Soda Glass)
B) Borosilicate Glass
C) Lead Glass
D) Other oxidized materials
- A) Soda Calcic Glass (Soda Glass)
Soda Calcic Glass is quite easy to mold, superior
in chemical resistance, the material is cheap and is commonly available.
This is the basic glass historically as well as technically. It is used
for sheet glass, bottles, etc.
- B) Borosilicate Glass
A glass contains borax B203. Its important characteristics are low expansion
coefficient and is chemically stable. In 1883, Shott first produced a new
optical glass for correcting color aberration.
- a) A low expansion coefficient
invented by Shott in 1883 whose expansion coefficient is approximately
60 x 10-7/degree C. As it has quite tough durability against thermal impacts,
it was used for the windshield of gas lantern for street lights invented
at that time set up a record of unprecedented sales volume and was used
until Thomas Edison invents an incandescent lamp. It draws keen interests
of engineers as the glass produced with new technology. Our touch-panel
is one of merchandises using this technique.
- b) PYREX
It was first developed by Corning Glass Works which was engaged in glass
bulb production, then began to supply storage battery container railway
signal system for Edison. In 1912, a borosilicate glass which has thermal
coefficient of 36 x 10-7/degree C, then 1915, PYREX Oven Ware were began
to sell as it withstands rapid heating as well asquick cooling.
- c) Glasses for Physical and Chemical Uses
As the World War I began, supplies to the United States from Shott of physical
and chemical use glass were suspended. It was proved to be a prototype
glass development stage of PYREX whose code number 7740 that it was superior
than the glass from Shott and Corning Glass Works began to supply it to
the market.
- d) Neutral Glass
This glass was first developed by Shott in 1910 for ampoule of injection
as the hydrogen ion density in the ampoule does not change for a long period
time, it was named then named neutral glass. Presently, ultra violet rays
absorption glass for ampoule is also available.
- e) Glass for Electron Tube
Tungsten, molybdenum and Kovar(28% Si, 17% Co, 54% Fe) are used for sealing
electron emitting electrode of vacuum tubes. Borosilicate glass is used
to seal such electrodes. Temperature coefficient of tungsten is 36 x 10-7/degree
C, molybdenum is 55 x 10-7/degree C and Kovar is 50 x 10-7/degree C. These
metals have similar temperature coefficient of borosilicate glass. If a
halogen(F2, C12, etc.) is emitted, it deteriorates the material used for
the cathode and reduces its thermal electron. As a general rule, a glass
which does not contain halogen is used for electron tubes.
For X-ray tubes, a Kovar glass is used which absorbs little X-ray.
f) 96 % Silicon Oxide Glass
In 1939, Corning Glass Works began to sell so called the Vycor, a High
Silicon Oxide Glass (96% Si O2). Vycor has a similar temperature coefficient
of quartz glass and is used for physical and chemical purposes, heat treatment
dish of fluorescent substance, mercury lamp, and sterilizing lamp tube,
which is a glass called the heat-resistant glass.
- C) Lead Glass for Optical Use
a) Crystal Glass
This glass is colorless and transparent and it looks like crystal is a
lead glass which contains 24 % or more lead (Pbo) by weight, and it has
a refractive index of more than 1.545% according to an international custom.
b) Lead Glass for Optical Use
This glass is used for lenses that require high refractive index and high
dispersion.
c) Lead Glass for Radiation Shield
This glass is used for X-ray shield. Pilkington of the U.K. and Nippon
Electric Glass are supplying this type of glass in Japan.
e) Glass for Electronics Use
Its crystalline flits are sealed with a funnel(funnel shaped glass) of
CRT(Cathode Ray Tube) for the purpose of shielding X-ray. Its advantage
over the other materials is to have a larger electrical resistance, and
low viscosity and low specific heat coefficient which enables to process
at a low temperature range, and low emission of gas as well. It fits for
the use of bulbs and X-ray tubes due to its good ability to shield X-ray.
- D) Other
Oxide Substance Glasses
- (1) Aluminosilicate Glass
Features : * Expansion Coefficient 30~60 x 10-7/degree C. * Water-resistant
in chemical endurance is extremely good. * Viscosity increases very rapidly
as the molten glass temperature falls down, therefore, it is good for production
of glass filament that requires short molding time. * Due to its softening
point is more 900 degrees C, it is good for use where high temperature
endurance is required. * A 25% higher than Soda Glass as far as stretch
strength is concerned.
a) The E Glass
The E glass is an aluminosilicate glass which is produced largest in volume.
It is used for FRP pipes.
b) The S Glass
It has a 33% higher stretch force and a 20% higher value of elasticity
than that of the E Glass, which is used for engine case of rocket, etc.
c) Glass Tube for Combustion
Due to its very high maximum usable temperature, it is used for kitchen
wares such as stove and percolator, high power electron tubes having very
high bulb temperatures, and halogen bulbs.
d) Photo Masking Glass
This glass is used for drawing pattern of integrated circuit.
(2) Borosilicate Glass
a) Used for soldering glass due to its low melting point and is used for
a bonding agent.
b) For windowpanes of neutron shielding of nuclear reactor.
(3) Orthophosphate Glass
a) For artificial bones of bio-ceramics and root of permanent teeth. More
and more use in the area of bio-ceramics are highly expected.
b) Promising future in the area of optical electronic laser. It is already
in practical use in the area of optical memories, for it shows a good violet
ray penetration, measurement of gamma rays of X-ray, and absorption of
near infrared ray.
Shott and Corning Glass Works say that
there are as many as 50,000 kinds of different composite glasses are available.
- * Ultrasonic Signal Delay Devices Made of Glass
- It is absolutely necessary for video cameras, video disks,
and satellite TV's.
A glass from Shott was first studied for radar use in 1940.
- * Faraday Rotation Glass
It is used for light isolator for optical communications as well as laser
nuclear fusion from now on.